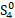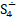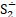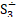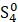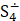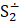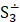what controls color in cinnabar?
Mineralogists,
Most cinnabar I've seen has a bright scarlet to deep red color, but this mineral can also be silvery-gray (with no red at all) and everything in-between. Seems a bit strange since this mineral has a pretty straightforward chemical formula. Does anyone know what controls this variation?
Thanks!
Kent Ratajeski
Kent Ratajeski, Ph.D.
301 Slone Research Building
Department of Earth and Environmental Sciences
University of Kentucky
Lexington, KY 40506-0053
Phone: 859-257-4444
Fax: 859-323-1938
http://www.as.uky.edu/users/krata2
Kent,
I have also seen this range of colors in cinnabar, from deep red to silvery. It is important to note, however, that even the silvery cinnabars maintain their deep red color when light is transmitted through the crystal.
My understanding of this variation is that cinnabar is slightly photosensitive. As it is exposed to light, over time, its surface will darken and become more silvery-gray than red. I suspect the silvery aspect is just the strongly adamantine luster becoming more prominent as the upper portions of the crystal darken, eliminating the red.
I’m interested to hear what others have to say on this topic!
Cheers,
Justin Bank
Justin Bank
MS Candidate
Miami University (Ohio)
Department of Geology and Environmental Earth Science
bankja@miamioh.edu
On Jun 4, 2023, at 11:33 AM, Ratajeski, Kent via MSA-talk msa-talk@minlists.org wrote:
Mineralogists,
Most cinnabar I've seen has a bright scarlet to deep red color, but this mineral can also be silvery-gray (with no red at all) and everything in-between. Seems a bit strange since this mineral has a pretty straightforward chemical formula. Does anyone know what controls this variation?
Thanks!
Kent Ratajeski
Kent Ratajeski, Ph.D.
301 Slone Research Building
Department of Earth and Environmental Sciences
University of Kentucky
Lexington, KY 40506-0053
Phone: 859-257-4444
Fax: 859-323-1938
http://www.as.uky.edu/users/krata2
MSA-talk mailing list -- msa-talk@minlists.org
To unsubscribe send an email to msa-talk-leave@minlists.org
It's my experience that the Chinese cinnabars on Quartz are dark and bright
red on dolomite. Hg has been found on silvery pieces, but the dark is
dismissed as light damaged with no experimental proof. There have been
suggestions that the dark is due to Se, but I'm unaware of any experimental
results. Interestingly the cinnabar from the Red Devil Mine In Alaska has
swirls of bright red and dark. I sent a sample to Chris Stanley and he
could detect no difference between the red and dark, other than what was
visible. If it is chemistry then the contaminants have so far been
undetectable.
On Sun, 4 Jun 2023 at 08:36, Ratajeski, Kent via MSA-talk <
msa-talk@minlists.org> wrote:
Mineralogists,
Most cinnabar I've seen has a bright scarlet to deep red color, but this
mineral can also be silvery-gray (with no red at all) and everything
in-between. Seems a bit strange since this mineral has a pretty
straightforward chemical formula. Does anyone know what controls this
variation?
Thanks!
Kent Ratajeski
Kent Ratajeski, Ph.D.
301 Slone Research Building
Department of Earth and Environmental Sciences
University of Kentucky
Lexington, KY 40506-0053
Phone: 859-257-4444
Fax: 859-323-1938
http://www.as.uky.edu/users/krata2
MSA-talk mailing list -- msa-talk@minlists.org
To unsubscribe send an email to msa-talk-leave@minlists.org
Hi Kent,
I wrote a blog post about this actually: https://mineralcare.web.ox.ac.uk/article/cinnabar
Cinnabar is naturally red, but can undergo photo-oxidiation to form colloidal metallic mercury at the crystal surface. It's the mercury that gives it the silver coloration.
Hope this helps!
Kathryn
Kathryn Royce
D.Phil Candidate, University of Oxford
Email: kathryn.royce@ouce.ox.ac.uk
Web: http://mineralcare.web.ox.ac.uk
https://mineralcare.super.site/
LinkedIn: https://www.linkedin.com/in/kathrynroyce/
------- Original Message -------
On Friday, June 2nd, 2023 at 20:41, Ratajeski, Kent via MSA-talk msa-talk@minlists.org wrote:
Mineralogists,
Most cinnabar I've seen has a bright scarlet to deep red color, but this mineral can also be silvery-gray (with no red at all) and everything in-between. Seems a bit strange since this mineral has a pretty straightforward chemical formula. Does anyone know what controls this variation?
Thanks!
Kent Ratajeski
Kent Ratajeski, Ph.D.
301 Slone Research Building
Department of Earth and Environmental Sciences
University of Kentucky
Lexington, KY 40506-0053
Phone: 859-257-4444
Fax: 859-323-1938
http://www.as.uky.edu/users/krata2
Dear Kent, Justin, et alia,
I think you may need to distinguish transmitted light color from
reflected light color. (As an aside, the ruby silvers--pyrargyrite,
proustite--are great examples of photosensitive minerals in an oxidizing
atmosphere. Maybe there is silver in some cinnabars?)
Good luck,
--Mary Johnson
On 6/4/2023 9:19 AM, Justin Bank via MSA-talk wrote:
Kent,
I have also seen this range of colors in cinnabar, from deep red to
silvery. It is important to note, however, that even the silvery
cinnabars maintain their deep red color when light is transmitted
through the crystal.
My understanding of this variation is that cinnabar is slightly
photosensitive. As it is exposed to light, over time, its surface will
darken and become more silvery-gray than red. I suspect the silvery
aspect is just the strongly adamantine luster becoming more prominent
as the upper portions of the crystal darken, eliminating the red.
I’m interested to hear what others have to say on this topic!
Cheers,
Justin Bank
Justin Bank
MS Candidate
Miami University (Ohio)
Department of Geology and Environmental Earth Science
bankja@miamioh.edu
On Jun 4, 2023, at 11:33 AM, Ratajeski, Kent via MSA-talk
msa-talk@minlists.org wrote:
Mineralogists,
Most cinnabar I've seen has a bright scarlet to deep red color, but
this mineral can also be silvery-gray (with no red at all) and
everything in-between. Seems a bit strange since this mineral has a
pretty straightforward chemical formula. Does anyone know what
controls this variation?
Thanks!
Kent Ratajeski
Kent Ratajeski, Ph.D.
301 Slone Research Building
Department of Earth and Environmental Sciences
University of Kentucky
Lexington, KY 40506-0053
Phone: 859-257-4444
Fax: 859-323-1938
http://www.as.uky.edu/users/krata2
MSA-talk mailing list -- msa-talk@minlists.org
To unsubscribe send an email to msa-talk-leave@minlists.org
MSA-talk mailing list --msa-talk@minlists.org
To unsubscribe send an email tomsa-talk-leave@minlists.org
I was thinking in the same direction in as far as cinnabar is concerned. Similar mechanisms play a role in the color change of some silver minerals, such as proustite.
On the other hand variability in color occurs also on hematitic iron ores.
Pure hematite ores are in some cases dull brownish red, some are steel gray with a strong metallic luster, and even brilliant red hematite microcrystals occur.
In the case of hematite the cause of the various colors is situated in the domain of texture, crystal size, porosity, grain size and all kinds of physical aspects of the material.
AFAIK the chemical composition does not play a big role in this case, except if the ore is not pure hematite, but rather a mixture of hematite, goethite and limonitic phases.
Or does someone have another explanation?
Greetings,
Rik Dillen - mailto:rik.dillen@skynet.be rik.dillen@skynet.be
Mineralogische Kring Antwerpen - http://www.minerant.org/ www.minerant.org
Lid worden van de MKA ? http://www.minerant.org/MKA/lidworden.html www.minerant.org/MKA/lidworden.html
MINERANT - 4-5 mei 2024
Antwerp Expo
From: K. Royce via MSA-talk msa-talk@minlists.org
Sent: Sunday, June 4, 2023 18:57
To: Ratajeski, Kent kent.ratajeski@uky.edu
Cc: MSA-talk msa-talk@minlists.org
Subject: [MSA-talk] Re: what controls color in cinnabar?
Hi Kent,
I wrote a blog post about this actually: https://mineralcare.web.ox.ac.uk/article/cinnabar
Cinnabar is naturally red, but can undergo photo-oxidiation to form colloidal metallic mercury at the crystal surface. It's the mercury that gives it the silver coloration.
Hope this helps!
Kathryn
Kathryn Royce
D.Phil Candidate, University of Oxford
Email: kathryn.royce@ouce.ox.ac.uk mailto:kathryn.royce@ouce.ox.ac.uk
Web: http://mineralcare.web.ox.ac.uk http://mineralcare.web.ox.ac.uk/
https://mineralcare.super.site/
LinkedIn: https://www.linkedin.com/in/kathrynroyce/
------- Original Message -------
On Friday, June 2nd, 2023 at 20:41, Ratajeski, Kent via MSA-talk <msa-talk@minlists.org mailto:msa-talk@minlists.org > wrote:
Mineralogists,
Most cinnabar I've seen has a bright scarlet to deep red color, but this mineral can also be silvery-gray (with no red at all) and everything in-between. Seems a bit strange since this mineral has a pretty straightforward chemical formula. Does anyone know what controls this variation?
Thanks!
Kent Ratajeski
Kent Ratajeski, Ph.D.
301 Slone Research Building
Department of Earth and Environmental Sciences
University of Kentucky
Lexington, KY 40506-0053
Phone: 859-257-4444
Fax: 859-323-1938
http://www.as.uky.edu/users/krata2
Hello,
As a cinnabar enthusiast and hunter of new mercury minerals, my compliments to the blog of Ms. Royce. The major studies are referenced and wonderfully summarized.
I have been to a large number of mercury mines. The color of cinnabar can vary from bright orange to nearly black across deposits. In my experience, cinnabar at volcanogenic mines is always more photoreactive than that found at carbonate-hosted mines. I also feel that exotic cinnabar colors are more often found at volcanogenic mines.
In addition to atomic chemistry causes, cinnabar can also change color depending on its grain size. This is an effect seen in the sub-micron range. It’s possible to change the color of vermillion pigment by controlling the grind. Orange colloidal cinnabar in silica is noteworthy at the McDermitt mine in Humboldt Co., Nevada, Manhattan mine near Clear Lake, CA, Kiggins mine near Portland, OR, and the Oriental mines near Chovar, Valencia, Spain. There are many others.
Cinnabar darkening and silvering impacts classical paintings. There is not just one cause of the darkening of classical art works, nor just one alteration product, and this also seems to be the case with natural mercury compounds found at mines. Setting aside the many compositional and structural causes of color, if there is any consistency with respect to darkened cinnabar that I have noticed, it is that the surface darkened or silvered by light exposure is often (but not always) a sub-micron layer that can be removed by abrasion.
I have personally witnessed color change in the field hundreds of times. It is curious that Nevada cinnabars nearly all darken in sunlight, and some in a matter of seconds, while Coast Range California cinnabars do not, or only do so very slowly. Again, the exception are volcanogenic California HgS deposits, such as at Clear Lake and the Manhattan mine nearby.
Of course, when it comes to minerals with mercury, nitrogen, and halogens, darkening should not be surprising. These mixture are basically a natural form of silver compounds used to make photographs, but with mercury being the dominant cation instead of silver. The chemistry of photographic film is discussed in this thesis: https://scholarcommons.sc.edu/cgi/viewcontent.cgi?article=1085 https://scholarcommons.sc.edu/cgi/viewcontent.cgi?article=1085&context=senior_theses#:~:text=Photographic%20paper%20and%20film%20consist,bromine%20is%20the%20most%20common &context=senior_theses#:~:text=Photographic%20paper%20and%20film%20consist,bromine%20is%20the%20most%20common.
Lastly, red orange terlinguacreekite (https://www.mindat.org/min-27470.html) from McDermitt darkens with light exposure. If the sample is them put away out of the light for several months, the original color returns. I sincerely hope that one day a competent lab might ascertain the mechanism for this color reversal. I believe it is possible that understanding terlinguacreekite color reversal might lead to a method to reverse cinnabar darkening in fine art works. BTW- I have washed massive New Almaden cinnabar in rust removers that turn it dark grey (nearly black). I was surprised to find the cinnabar reverted to its original color after being kept in the dark for some time, just like terlinguacreekite.
Fun!
Cheers,
Mike
Michael F. Cox
Vice President
New Almaden Quicksilver County Park Association
PO Box 124, New Almaden, CA 95042-0124
(408) 644-7848 (cell)
mailto:mercury_miner@netzero.net mercury_miner@netzero.net
mailto:mercury.miner@gmail.com mercury.miner@gmail.com
From: K. Royce via MSA-talk msa-talk@minlists.org
Sent: Sunday, June 4, 2023 9:57 AM
To: Ratajeski, Kent kent.ratajeski@uky.edu
Cc: MSA-talk msa-talk@minlists.org
Subject: [MSA-talk] Re: what controls color in cinnabar?
Hi Kent,
I wrote a blog post about this actually: https://mineralcare.web.ox.ac.uk/article/cinnabar
Cinnabar is naturally red, but can undergo photo-oxidiation to form colloidal metallic mercury at the crystal surface. It's the mercury that gives it the silver coloration.
Hope this helps!
Kathryn
Kathryn Royce
D.Phil Candidate, University of Oxford
Email: kathryn.royce@ouce.ox.ac.uk mailto:kathryn.royce@ouce.ox.ac.uk
Web: http://mineralcare.web.ox.ac.uk http://mineralcare.web.ox.ac.uk/
https://mineralcare.super.site/
LinkedIn: https://www.linkedin.com/in/kathrynroyce/
------- Original Message -------
On Friday, June 2nd, 2023 at 20:41, Ratajeski, Kent via MSA-talk <msa-talk@minlists.org mailto:msa-talk@minlists.org > wrote:
Mineralogists,
Most cinnabar I've seen has a bright scarlet to deep red color, but this mineral can also be silvery-gray (with no red at all) and everything in-between. Seems a bit strange since this mineral has a pretty straightforward chemical formula. Does anyone know what controls this variation?
Thanks!
Kent Ratajeski
Kent Ratajeski, Ph.D.
301 Slone Research Building
Department of Earth and Environmental Sciences
University of Kentucky
Lexington, KY 40506-0053
Phone: 859-257-4444
Fax: 859-323-1938
http://www.as.uky.edu/users/krata2
Good morning,
It may be worth noting that a tenebrescent variety of sodalite called hackmanite exhibits a certain degree of reversible photsensitivity.
Freshly broken surfaces exhibit a brilliant magenta color, which typically fades to a dull grey within minutes. Vredenburg (1904) described it thus, "The phenomenon is particularly brilliant when the rock is first broken in the field, and the large blocks of elæolite (some of which are over a yard wide) appear, on fracture, as if suffused with blood." If the specimen is kept under fluorescent light or in darkness for several days, a purplish color returns, though it is generally darker, less red, and more subdued.
From a crystal chemical perspective, hackmanite relates to sodalite (ideally, Na8Al6Si6O24Cl2) through substitution of sulfur for chlorine, likely coupled with ferric iron substituting for aluminum. This was suggested by Petersen (1983) as a basis for the photochemical behavior of hackmanite, and hackmanite that I analyzed from Mont Saint-Hilaire has a chemistry that is consistent with that substitution.
But the particulars of the mechanism are likely due not just to the presence of sulfur but of the presence of sulfur radicals. In sodalite, [cid:image001.png@01D99794.2960E430] radicals promote the characteristic blue color of the mineral, with the occasional presence of [cid:image002.png@01D99794.2960E430] radicals that produce a yellow coloration. In hackmanite, some combination of [cid:image003.png@01D99794.2960E430] and [cid:image004.png@01D99794.2960E430] , which produce a red coloration, probably occur in combination with [cid:image002.png@01D99794.2960E430] (and perhaps [cid:image001.png@01D99794.2960E430] ) to yield the purple color. (Clark & Cobbold, 1978; Gobeltz-Hautecoeur et al., 2002) The photosensitivity then probably relates to electron transfer driven by absorption of light.
Being that cinnabar is sulfur-essential, it seems likely that there would be some inclusion of sulfur radicals in its structure, as well, which might explain observed photosensitive behavior in that species.
I hope that that is useful.
Regards,
Peter Tice
Math Teacher
Rivendell Academy
Orford, New Hampshire 03777
From: mercury_miner--- via MSA-talk msa-talk@minlists.org
Sent: Sunday, June 4, 2023 4:57 PM
To: 'K. Royce' kroyce@pm.me; 'Ratajeski, Kent' kent.ratajeski@uky.edu
Cc: 'MSA-talk' msa-talk@minlists.org
Subject: [MSA-talk] Re: what controls color in cinnabar?
[*This email is from an outside sender. Please be careful clicking on links and attachments.]
Hello,
As a cinnabar enthusiast and hunter of new mercury minerals, my compliments to the blog of Ms. Royce. The major studies are referenced and wonderfully summarized.
I have been to a large number of mercury mines. The color of cinnabar can vary from bright orange to nearly black across deposits. In my experience, cinnabar at volcanogenic mines is always more photoreactive than that found at carbonate-hosted mines. I also feel that exotic cinnabar colors are more often found at volcanogenic mines.
In addition to atomic chemistry causes, cinnabar can also change color depending on its grain size. This is an effect seen in the sub-micron range. It's possible to change the color of vermillion pigment by controlling the grind. Orange colloidal cinnabar in silica is noteworthy at the McDermitt mine in Humboldt Co., Nevada, Manhattan mine near Clear Lake, CA, Kiggins mine near Portland, OR, and the Oriental mines near Chovar, Valencia, Spain. There are many others.
Cinnabar darkening and silvering impacts classical paintings. There is not just one cause of the darkening of classical art works, nor just one alteration product, and this also seems to be the case with natural mercury compounds found at mines. Setting aside the many compositional and structural causes of color, if there is any consistency with respect to darkened cinnabar that I have noticed, it is that the surface darkened or silvered by light exposure is often (but not always) a sub-micron layer that can be removed by abrasion.
I have personally witnessed color change in the field hundreds of times. It is curious that Nevada cinnabars nearly all darken in sunlight, and some in a matter of seconds, while Coast Range California cinnabars do not, or only do so very slowly. Again, the exception are volcanogenic California HgS deposits, such as at Clear Lake and the Manhattan mine nearby.
Of course, when it comes to minerals with mercury, nitrogen, and halogens, darkening should not be surprising. These mixture are basically a natural form of silver compounds used to make photographs, but with mercury being the dominant cation instead of silver. The chemistry of photographic film is discussed in this thesis: https://scholarcommons.sc.edu/cgi/viewcontent.cgi?article=1085&context=senior_theses#:~:text=Photographic%20paper%20and%20film%20consist,bromine%20is%20the%20most%20common.
Lastly, red orange terlinguacreekite (https://www.mindat.org/min-27470.html) from McDermitt darkens with light exposure. If the sample is them put away out of the light for several months, the original color returns. I sincerely hope that one day a competent lab might ascertain the mechanism for this color reversal. I believe it is possible that understanding terlinguacreekite color reversal might lead to a method to reverse cinnabar darkening in fine art works. BTW- I have washed massive New Almaden cinnabar in rust removers that turn it dark grey (nearly black). I was surprised to find the cinnabar reverted to its original color after being kept in the dark for some time, just like terlinguacreekite.
Fun!
Cheers,
Mike
Michael F. Cox
Vice President
New Almaden Quicksilver County Park Association
PO Box 124, New Almaden, CA 95042-0124
(408) 644-7848 (cell)
mercury_miner@netzero.netmailto:mercury_miner@netzero.net
mercury.miner@gmail.commailto:mercury.miner@gmail.com
From: K. Royce via MSA-talk <msa-talk@minlists.orgmailto:msa-talk@minlists.org>
Sent: Sunday, June 4, 2023 9:57 AM
To: Ratajeski, Kent <kent.ratajeski@uky.edumailto:kent.ratajeski@uky.edu>
Cc: MSA-talk <msa-talk@minlists.orgmailto:msa-talk@minlists.org>
Subject: [MSA-talk] Re: what controls color in cinnabar?
Hi Kent,
I wrote a blog post about this actually: https://mineralcare.web.ox.ac.uk/article/cinnabar
Cinnabar is naturally red, but can undergo photo-oxidiation to form colloidal metallic mercury at the crystal surface. It's the mercury that gives it the silver coloration.
Hope this helps!
Kathryn
Kathryn Royce
D.Phil Candidate, University of Oxford
Email: kathryn.royce@ouce.ox.ac.ukmailto:kathryn.royce@ouce.ox.ac.uk
Web: http://mineralcare.web.ox.ac.ukhttp://mineralcare.web.ox.ac.uk/
https://mineralcare.super.site/
LinkedIn: https://www.linkedin.com/in/kathrynroyce/
------- Original Message -------
On Friday, June 2nd, 2023 at 20:41, Ratajeski, Kent via MSA-talk <msa-talk@minlists.orgmailto:msa-talk@minlists.org> wrote:
Mineralogists,
Most cinnabar I've seen has a bright scarlet to deep red color, but this mineral can also be silvery-gray (with no red at all) and everything in-between. Seems a bit strange since this mineral has a pretty straightforward chemical formula. Does anyone know what controls this variation?
Thanks!
Kent Ratajeski
Kent Ratajeski, Ph.D.
301 Slone Research Building
Department of Earth and Environmental Sciences
University of Kentucky
Lexington, KY 40506-0053
Phone: 859-257-4444
Fax: 859-323-1938
http://www.as.uky.edu/users/krata2
Disclaimer: This email may contain information protected under the Family Educational Rights and Privacy Act and/or confidentiality requirements. Unauthorized use, disclosure, or copying is strictly prohibited and may be unlawful. If this email contains student information and you are not entitled to access such information under FERPA, federal regulations require that you destroy this email without reviewing it and you may not forward it to anyone. If you have received this communication in error, please notify sender immediately.
Hello,
Extremely useful! Thank you so very much for the generous information and
the reminder about the more complex electron transfer between radicals,
especially sulfur.
I have no clue, but I wonder if the presence of chlorine somehow contributes
to the darkening intensity or speed? In a mine pit back in 2017 or so, I
broke open a large piece of cinnabar-corderoite ore in the presence of
others. The surface facing the sun went from brilliant red to nearly black
in about 15 seconds. We noted an odor like hypochlorite bleach while the
reaction was occurring.
Best regards,
Mike
Michael F. Cox
PO Box 786, Soquel, CA 95073-0786
(408) 644-7848 (cell)
(831) 462-1907 (home)
From: Peter Tice PTice@RivendellSchool.org
Sent: Monday, June 5, 2023 6:58 AM
To: mercury_miner@netzero.net; 'K. Royce' kroyce@pm.me; 'Ratajeski, Kent'
kent.ratajeski@uky.edu
Cc: 'MSA-talk' msa-talk@minlists.org
Subject: RE: [MSA-talk] Re: what controls color in cinnabar?
Good morning,
It may be worth noting that a tenebrescent variety of sodalite called
hackmanite exhibits a certain degree of reversible photsensitivity.
Freshly broken surfaces exhibit a brilliant magenta color, which typically
fades to a dull grey within minutes. Vredenburg (1904) described it thus,
The phenomenon is particularly brilliant when the rock is first broken in
the field, and the large blocks of elæolite (some of which are over a yard
wide) appear, on fracture, as if suffused with blood. If the specimen is
kept under fluorescent light or in darkness for several days, a purplish
color returns, though it is generally darker, less red, and more subdued.
From a crystal chemical perspective, hackmanite relates to sodalite
(ideally, Na8Al6Si6O24Cl2) through substitution of sulfur for chlorine,
likely coupled with ferric iron substituting for aluminum. This was
suggested by Petersen (1983) as a basis for the photochemical behavior of
hackmanite, and hackmanite that I analyzed from Mont Saint-Hilaire has a
chemistry that is consistent with that substitution.
But the particulars of the mechanism are likely due not just to the presence
of sulfur but of the presence of sulfur radicals. In sodalite, radicals
promote the characteristic blue color of the mineral, with the occasional
presence of radicals that produce a yellow coloration. In hackmanite, some
combination of and, which produce a red coloration, probably occur in
combination with (and perhaps) to yield the purple color. (Clark & Cobbold,
1978; Gobeltz-Hautecoeur et al., 2002) The photosensitivity then probably
relates to electron transfer driven by absorption of light.
Being that cinnabar is sulfur-essential, it seems likely that there would be
some inclusion of sulfur radicals in its structure, as well, which might
explain observed photosensitive behavior in that species.
I hope that that is useful.
Regards,
Peter Tice
Math Teacher
Rivendell Academy
Orford, New Hampshire 03777
From: mercury_miner--- via MSA-talk <msa-talk@minlists.org
mailto:msa-talk@minlists.org >
Sent: Sunday, June 4, 2023 4:57 PM
To: 'K. Royce' <kroyce@pm.me mailto:kroyce@pm.me >; 'Ratajeski, Kent'
<kent.ratajeski@uky.edu mailto:kent.ratajeski@uky.edu >
Cc: 'MSA-talk' <msa-talk@minlists.org mailto:msa-talk@minlists.org >
Subject: [MSA-talk] Re: what controls color in cinnabar?
[*This email is from an outside sender. Please be careful clicking on links
and attachments.]
Hello,
As a cinnabar enthusiast and hunter of new mercury minerals, my compliments
to the blog of Ms. Royce. The major studies are referenced and wonderfully
summarized.
I have been to a large number of mercury mines. The color of cinnabar can
vary from bright orange to nearly black across deposits. In my experience,
cinnabar at volcanogenic mines is always more photoreactive than that found
at carbonate-hosted mines. I also feel that exotic cinnabar colors are more
often found at volcanogenic mines.
In addition to atomic chemistry causes, cinnabar can also change color
depending on its grain size. This is an effect seen in the sub-micron range.
Its possible to change the color of vermillion pigment by controlling the
grind. Orange colloidal cinnabar in silica is noteworthy at the McDermitt
mine in Humboldt Co., Nevada, Manhattan mine near Clear Lake, CA, Kiggins
mine near Portland, OR, and the Oriental mines near Chovar, Valencia, Spain.
There are many others.
Cinnabar darkening and silvering impacts classical paintings. There is not
just one cause of the darkening of classical art works, nor just one
alteration product, and this also seems to be the case with natural mercury
compounds found at mines. Setting aside the many compositional and
structural causes of color, if there is any consistency with respect to
darkened cinnabar that I have noticed, it is that the surface darkened or
silvered by light exposure is often (but not always) a sub-micron layer that
can be removed by abrasion.
I have personally witnessed color change in the field hundreds of times. It
is curious that Nevada cinnabars nearly all darken in sunlight, and some in
a matter of seconds, while Coast Range California cinnabars do not, or only
do so very slowly. Again, the exception are volcanogenic California HgS
deposits, such as at Clear Lake and the Manhattan mine nearby.
Of course, when it comes to minerals with mercury, nitrogen, and halogens,
darkening should not be surprising. These mixture are basically a natural
form of silver compounds used to make photographs, but with mercury being
the dominant cation instead of silver. The chemistry of photographic film is
discussed in this thesis:
https://scholarcommons.sc.edu/cgi/viewcontent.cgi?article=1085
<https://scholarcommons.sc.edu/cgi/viewcontent.cgi?article=1085&context=seni
or_theses#:~:text=Photographic%20paper%20and%20film%20consist,bromine%20is%2
0the%20most%20common>
&context=senior_theses#:~:text=Photographic%20paper%20and%20film%20consist,b
romine%20is%20the%20most%20common.
Lastly, red orange terlinguacreekite (https://www.mindat.org/min-27470.html)
from McDermitt darkens with light exposure. If the sample is them put away
out of the light for several months, the original color returns. I sincerely
hope that one day a competent lab might ascertain the mechanism for this
color reversal. I believe it is possible that understanding
terlinguacreekite color reversal might lead to a method to reverse cinnabar
darkening in fine art works. BTW- I have washed massive New Almaden cinnabar
in rust removers that turn it dark grey (nearly black). I was surprised to
find the cinnabar reverted to its original color after being kept in the
dark for some time, just like terlinguacreekite.
Fun!
Cheers,
Mike
Michael F. Cox
Vice President
New Almaden Quicksilver County Park Association
PO Box 124, New Almaden, CA 95042-0124
(408) 644-7848 (cell)
mailto:mercury_miner@netzero.net mercury_miner@netzero.net
mailto:mercury.miner@gmail.com mercury.miner@gmail.com
From: K. Royce via MSA-talk <msa-talk@minlists.org
mailto:msa-talk@minlists.org >
Sent: Sunday, June 4, 2023 9:57 AM
To: Ratajeski, Kent <kent.ratajeski@uky.edu mailto:kent.ratajeski@uky.edu
Cc: MSA-talk <msa-talk@minlists.org mailto:msa-talk@minlists.org >
Subject: [MSA-talk] Re: what controls color in cinnabar?
Hi Kent,
I wrote a blog post about this actually:
https://mineralcare.web.ox.ac.uk/article/cinnabar
https://mineralcare.web.ox.ac.uk/article/cinnabar
Cinnabar is naturally red, but can undergo photo-oxidiation to form
colloidal metallic mercury at the crystal surface. It's the mercury that
gives it the silver coloration.
Hope this helps!
Kathryn
Kathryn Royce
D.Phil Candidate, University of Oxford
Email: mailto:kathryn.royce@ouce.ox.ac.uk kathryn.royce@ouce.ox.ac.uk
Web: http://mineralcare.web.ox.ac.uk/ http://mineralcare.web.ox.ac.uk
<https://mineralcare.super.site/>
https://mineralcare.super.site/
LinkedIn: https://www.linkedin.com/in/kathrynroyce/
https://www.linkedin.com/in/kathrynroyce/
------- Original Message -------
On Friday, June 2nd, 2023 at 20:41, Ratajeski, Kent via MSA-talk
<msa-talk@minlists.org mailto:msa-talk@minlists.org > wrote:
Mineralogists,
Most cinnabar I've seen has a bright scarlet to deep red color, but this
mineral can also be silvery-gray (with no red at all) and everything
in-between. Seems a bit strange since this mineral has a pretty
straightforward chemical formula. Does anyone know what controls this
variation?
Thanks!
Kent Ratajeski
Kent Ratajeski, Ph.D.
301 Slone Research Building
Department of Earth and Environmental Sciences
University of Kentucky
Lexington, KY 40506-0053
Phone: 859-257-4444
Fax: 859-323-1938
http://www.as.uky.edu/users/krata2 http://www.as.uky.edu/users/krata2
Disclaimer: This email may contain information protected under the Family
Educational Rights and Privacy Act and/or confidentiality requirements.
Unauthorized use, disclosure, or copying is strictly prohibited and may be
unlawful. If this email contains student information and you are not
entitled to access such information under FERPA, federal regulations require
that you destroy this email without reviewing it and you may not forward it
to anyone. If you have received this communication in error, please notify
sender immediately.